We develop innovative biopharmaceuticals based on stem cells for pet animals. We belong to a small group of pioneers that create market standards. The first veterinary biopharmaceutical was registered by the European Medicines Agency in 2017. Currently the interest of global pharmaceutical companies in veterinary biopharmaceuticals is evident. This will greatly facilitate the implementation of our business model.
Bioceltix is implementing an animal-safe technology for the therapeutic use of allogeneic stem cells (one donor – many recipients), characterised by business scalability, as the first company in Europe.


We are the first company in Europe:
Our technology for the procurement and in vitro cultivation of allogeneic stem cells (one donor – many recipients), as well as advanced procedures of quality control, both ensure safe use of our medicines by eliminating the risk of rejection by the recipient immune system.
Furthermore, the risk of neoplastic transformations affecting stem cells intended for clinical purposes has also been eliminated, owing to the implementation of genetic research being so far unprecedented in the veterinary science.
Products by Bioceltix make use of the anti-inflammatory and analgesic properties of mesenchymal stem cells. They constitute a modern alternative to the symptomatic pharmacological treatment that is mostly based on conventional (chemical) analgesics, as well as non-steroidal and steroidal anti-inflammatory drugs whose long-term use may entail numerous side effects.
Contrary to conventional chemical drugs, biopharmaceuticals developed by Bioceltix have a long-term action based on natural processes occurring within the organism, due to which they do not induce any side effects. Additionally, they are capable of actively supporting the reconstruction of tissues damaged by a disease, demonstrating strong proregenerative action.

Both the portfolio of original products by Bioceltix and the R&D works it conducts reflect global trends which will considerably facilitate the implementation of the business model it has adopted. The development strategy of world’s largest veterinary companies includes conducting research on new medicines mostly with regard to the field of diseases affecting the locomotor system (diseases of the joints), autoimmune diseases (allergies, atopic dermatitis) and tumours.
|
Target group |
Code | Therapeutic area | Planned initiation of clinical trials |
| BCX-CM-J | Degenerative changes of joints | October of 2019 | |
| BCX-CM-S | Generalised osteoarthritis, autoimmune diseases | October of 2019 | |
| BCX-CM-AD | Atopic dermatitis | January of 2020 | |
| BCX-EM | Tendon damage, complex bone fractures | May of 2020 | |
| BCX-FM | Chronic feline gingivostomatitis | November of 2020 |
The portfolio of our products will be systematically expanded. We mostly create the company’s value through the development of innovative veterinary products within the framework of own R&D effort that currently encompasses the following, among others:
the development of a rapid test strip for the diagnosis of arthritis in dogs, based on a caninised monoclonal antibody and intended for unassisted use by a veterinary doctor – currently, the diagnostics is ambiguous and complex, being based on interviews with the pet owner, roentgenography and MRI that are both expensive and strenuous for patients,
the development of a new drug for canine lymphoma and leukemia, as based on a conjugate of a caninised monoclonal antibody with a cytostatic agent being applied in targeted therapy where the administered medicine will only be toxic for cancerous cells, contrary to conventional and systemic administration of cytostatics that are also toxic for healthy cells,
the development of technology for the isolation of stem cells from the foetal tissue (the placenta, umbilical cord) of dogs and horses – such cells are more of a primary nature, demonstrating a higher proliferation potential, being identical in terms of their biological age, exemplifying low immunogenicity and a natural capability of being differentiated into neurons,
the development of technology for the lyophilisation of stem cells in a manner making it possible for enlivening them before they are administered to the patient – the new technology will allow for storing stem cells in conditions that do not require deep freezing, extending their shelf-life and producing new forms of medicines.

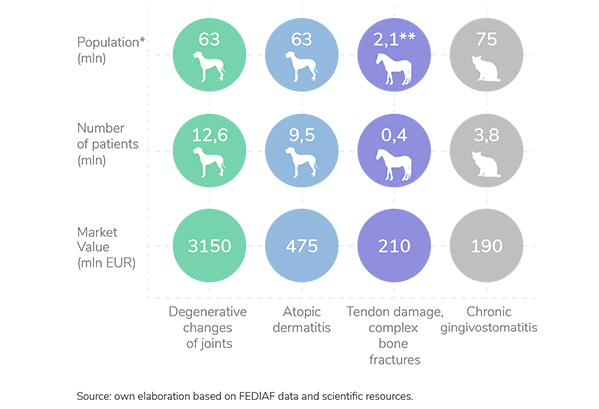
* All data refer to the European Union
** Light, recreational and accompanying horses included only
The business model of Bioceltix is based on:

